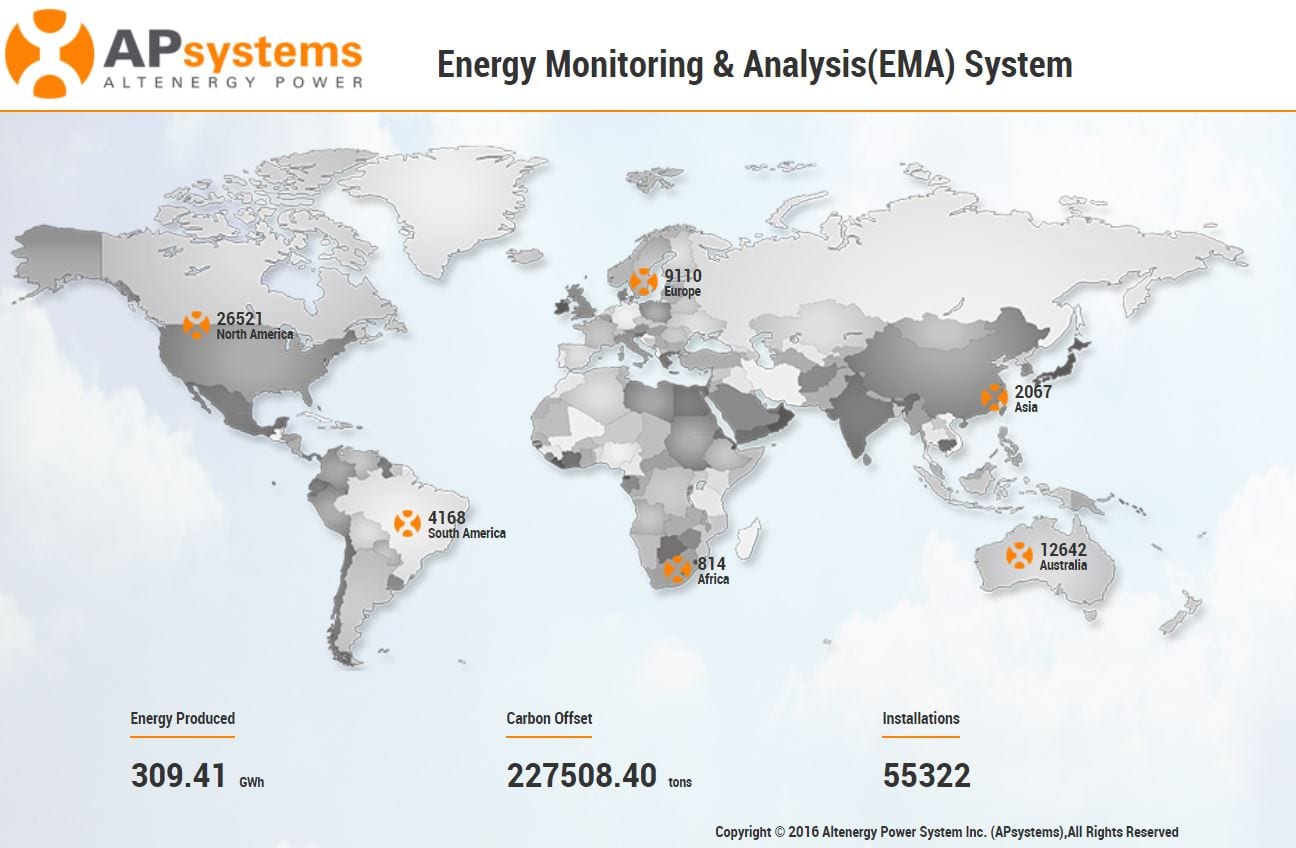
Risk-Based Biologics: CMC Flexibilities in the EU Regulatory System - BioProcess InternationalBioProcess International
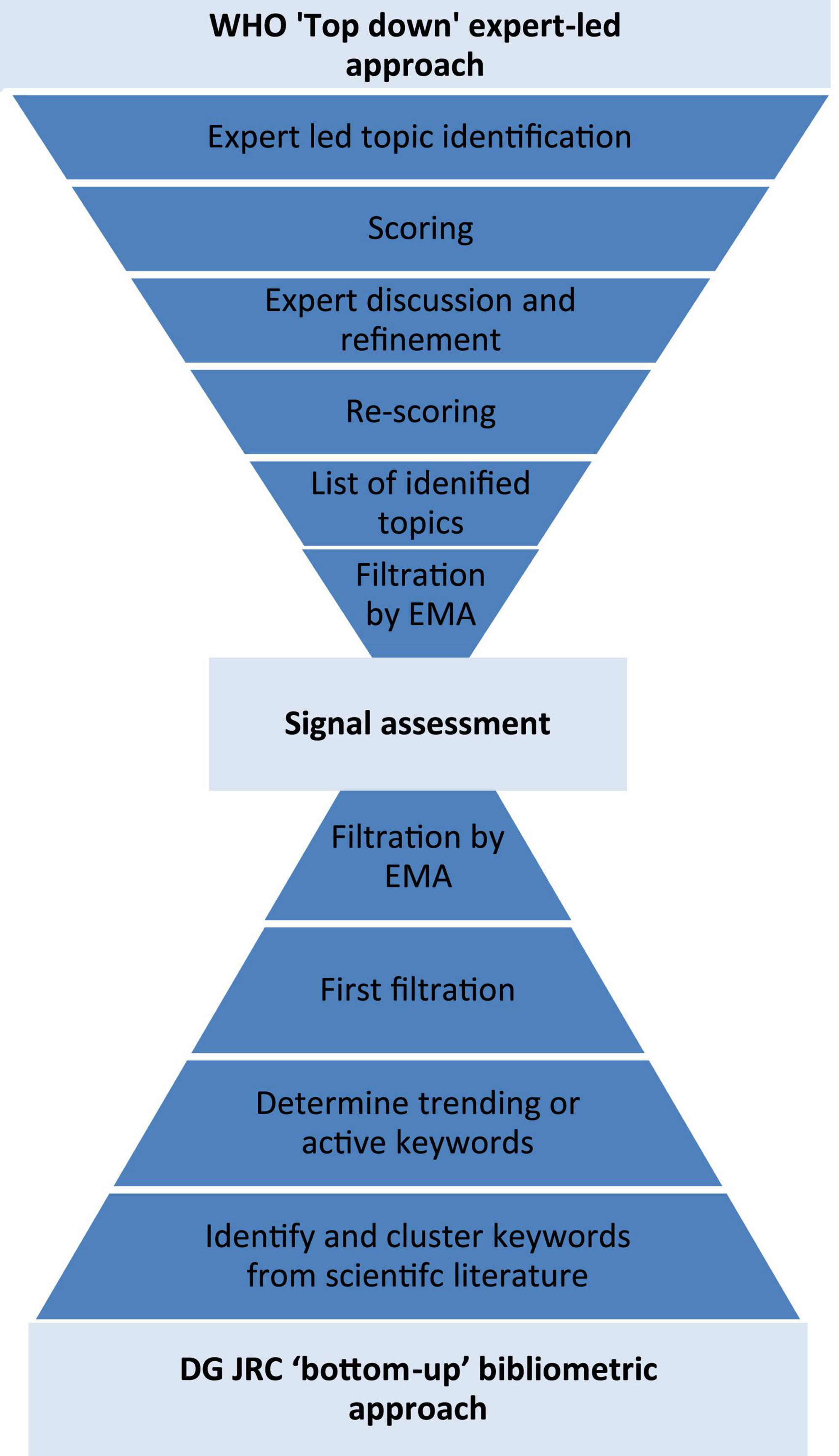
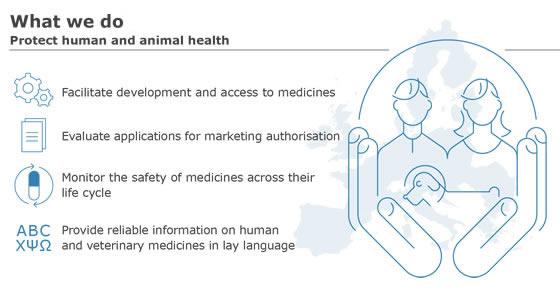
Frontiers | Health horizons: Future trends and technologies from the European Medicines Agency's horizon scanning collaborations

Data Safety and Monitoring Boards Should Be Required for Both Early- and Late-Phase Clinical Trials - ScienceDirect

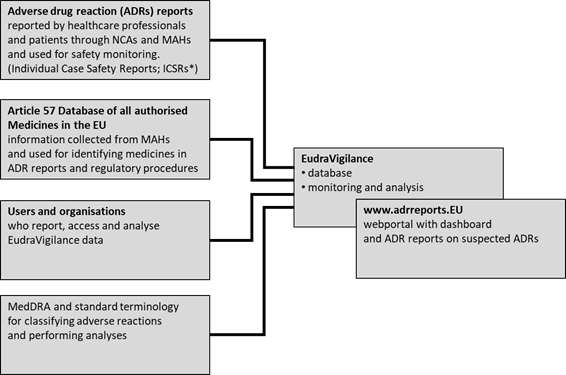
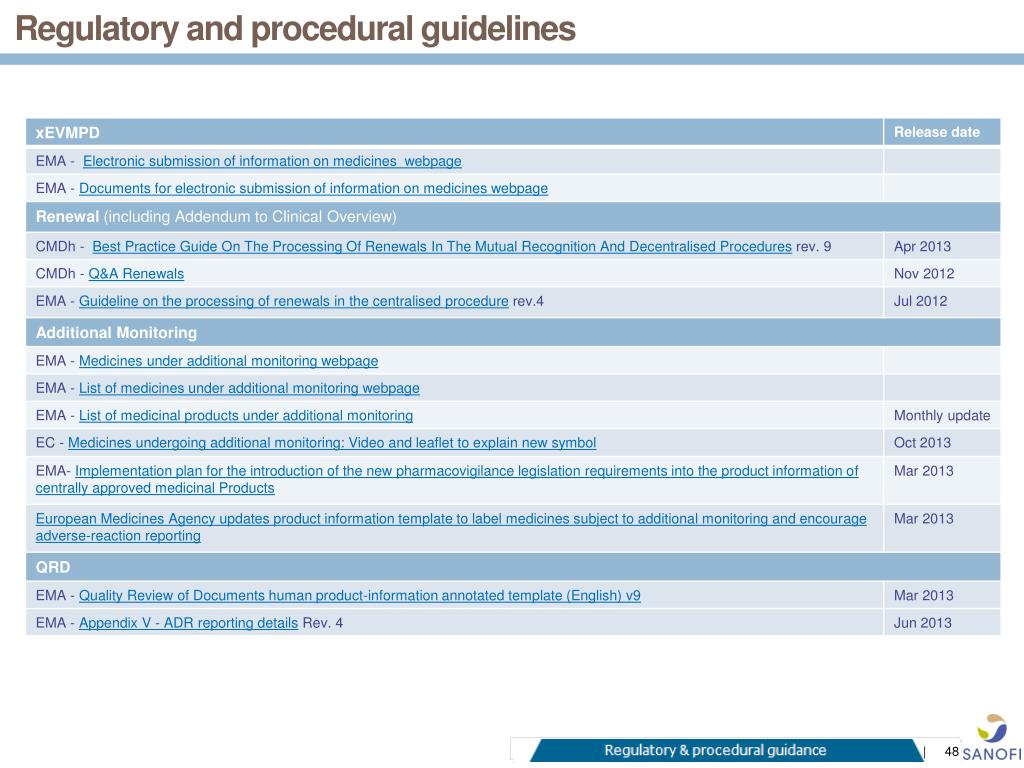
TAIEX Workshop on the Implementation of EU Pharmacovigilance Legislation - BELGRADE CLAUDIA PANAIT TAIEX Expert – European Commission Legal Adviser. - ppt download
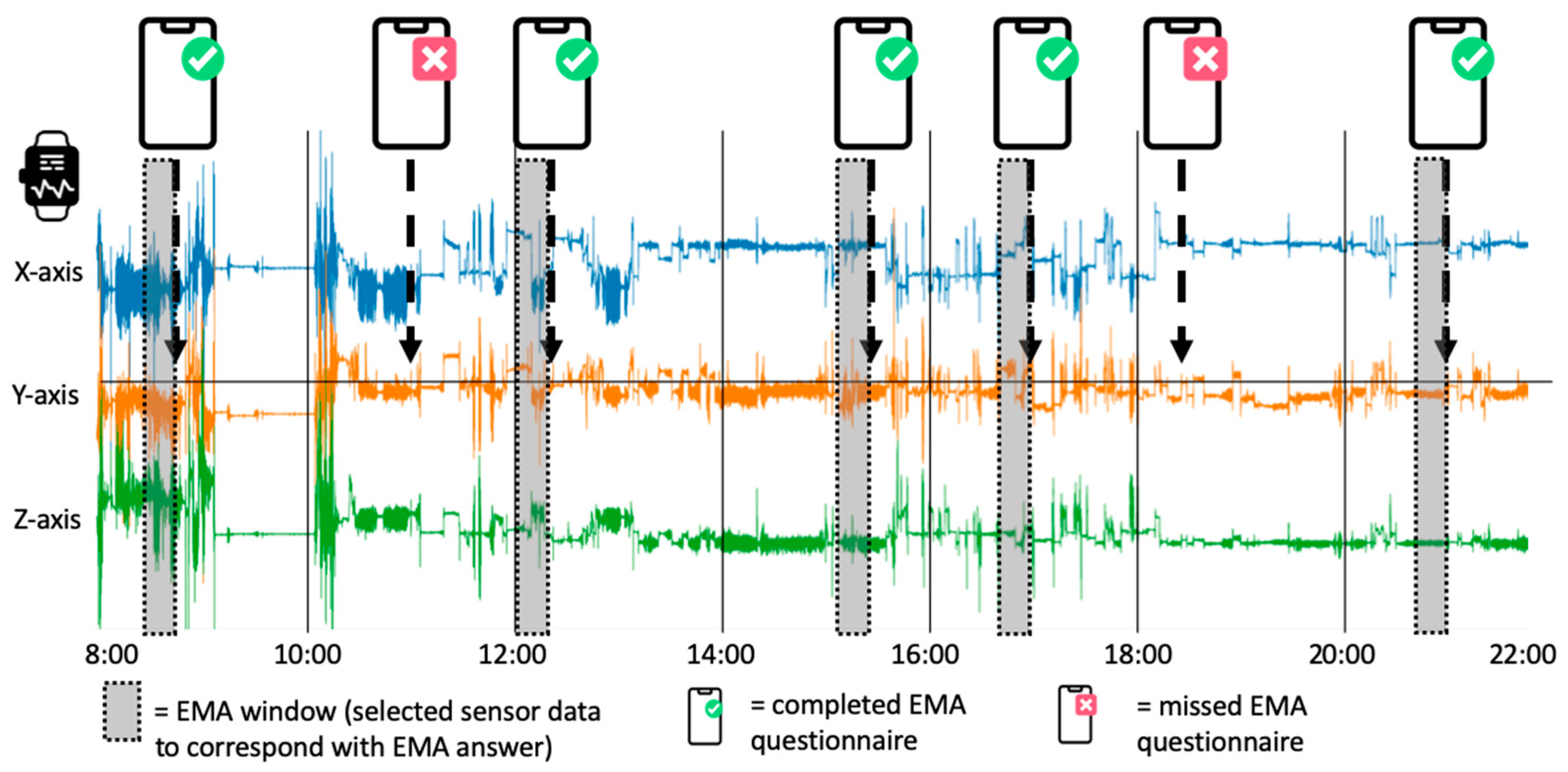
Data | Free Full-Text | A Long-Term, Real-Life Parkinson Monitoring Database Combining Unscripted Objective and Subjective Recordings

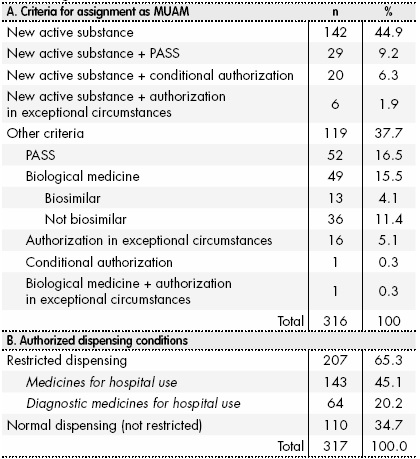
Evaluation of quantitative signal detection in EudraVigilance for orphan drugs: possible risk of false negatives | Semantic Scholar